
for the EP & Cath Lab

CLARAVUE® features the only disposable, prewired patient monitoring kit — the radiolucent design provides the best-in-class for highest quality traces in Electrophysiology and Cardiology care. CLARAVUE® works with a Bioactive Adapter Trunk Cable. Fully repositionable, CLARAVUE® provides a 5-day wear time. Our 12 Lead Patient Kit is perfect for patients moving from EP/Cath to Vis. Simply remove the V lead set and keep on the limb leads.

Claravue Features
Invisible Wires & Electrodes
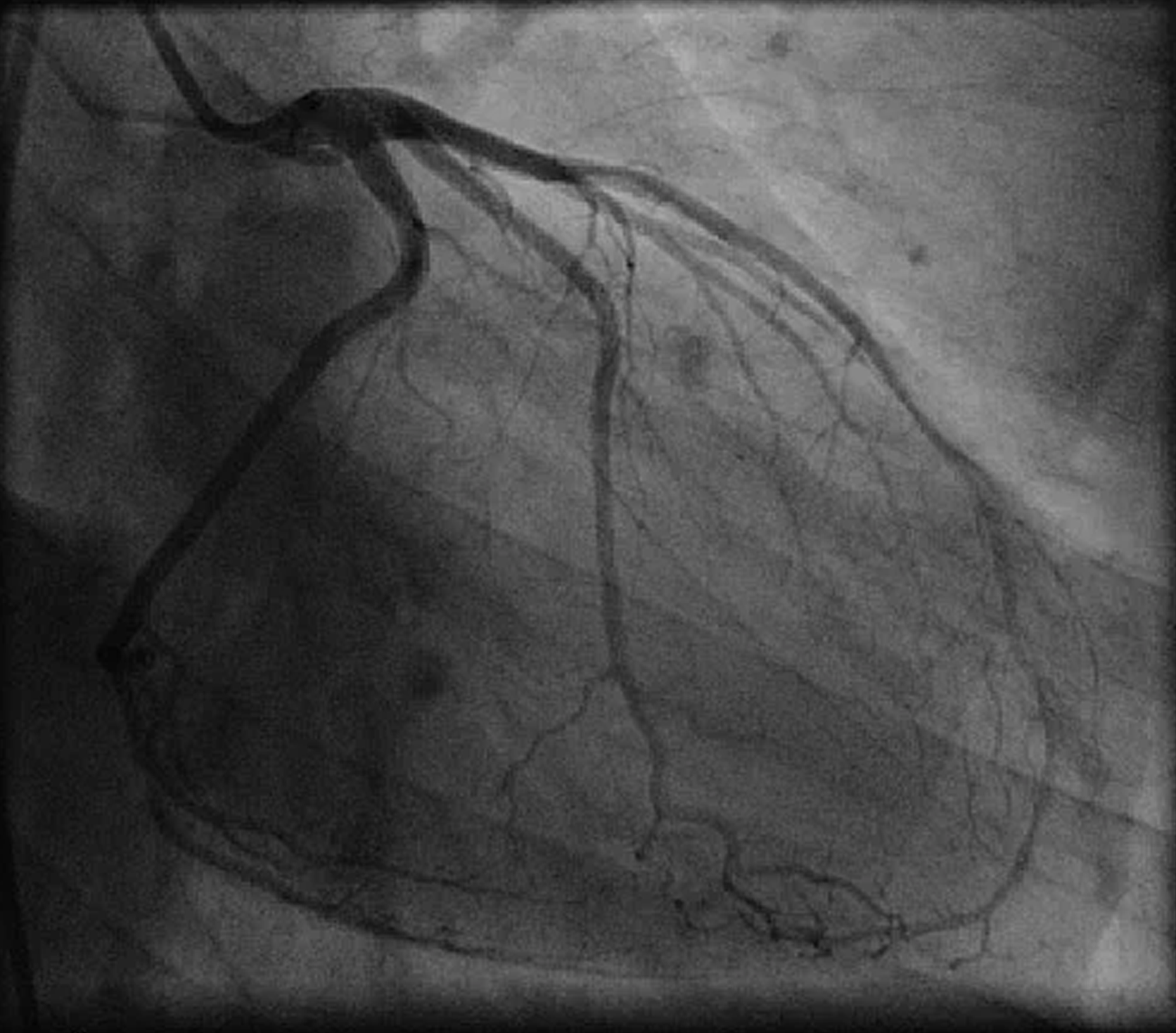
No image obstruction under x-ray and fluoroscopy
Invisible under x-ray and fluoroscopy, CLARAVUE’s radiolucent leadwires allow for outstanding imaging during procedures in the Catherization Lab or Electrophysiology Department. Notably, CLARAVUE’s carbon wiring of electrodes does not appear under imaging equipment.
Disposable & Hygienic

Single Patient Use - Up to 5 Day Wear Time
Each completely disposable Patient Kit (paired with the antimicrobial effect of reusable bioactive, metalloacid-coated cables) ensures the most hygienic solution for infection control.
Quality Trace

Pre-wired electrodes eliminate the need to snap leadwires onto electrodes.
CLARAVUE’S best-in-class electrodes reduce motion artifact associated with movement of the leadwires on electrode snaps. Repositionable, disposable patient kits can be used for up to 5-days.
Quick Patient Hookup

Patented quick connection point, repositionable and comfortable
CLARAVUE’S patented quick connection consolidates standard 10 leadwire ends to two simple, plug-style connections. Reduce opportunities for error by simplifying connections. Lightweight and conforming cables increase patient comfort.
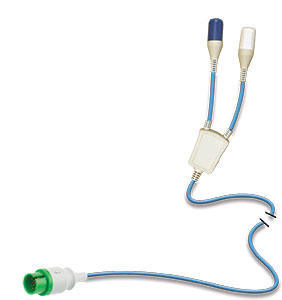
The CLARAVUE® System
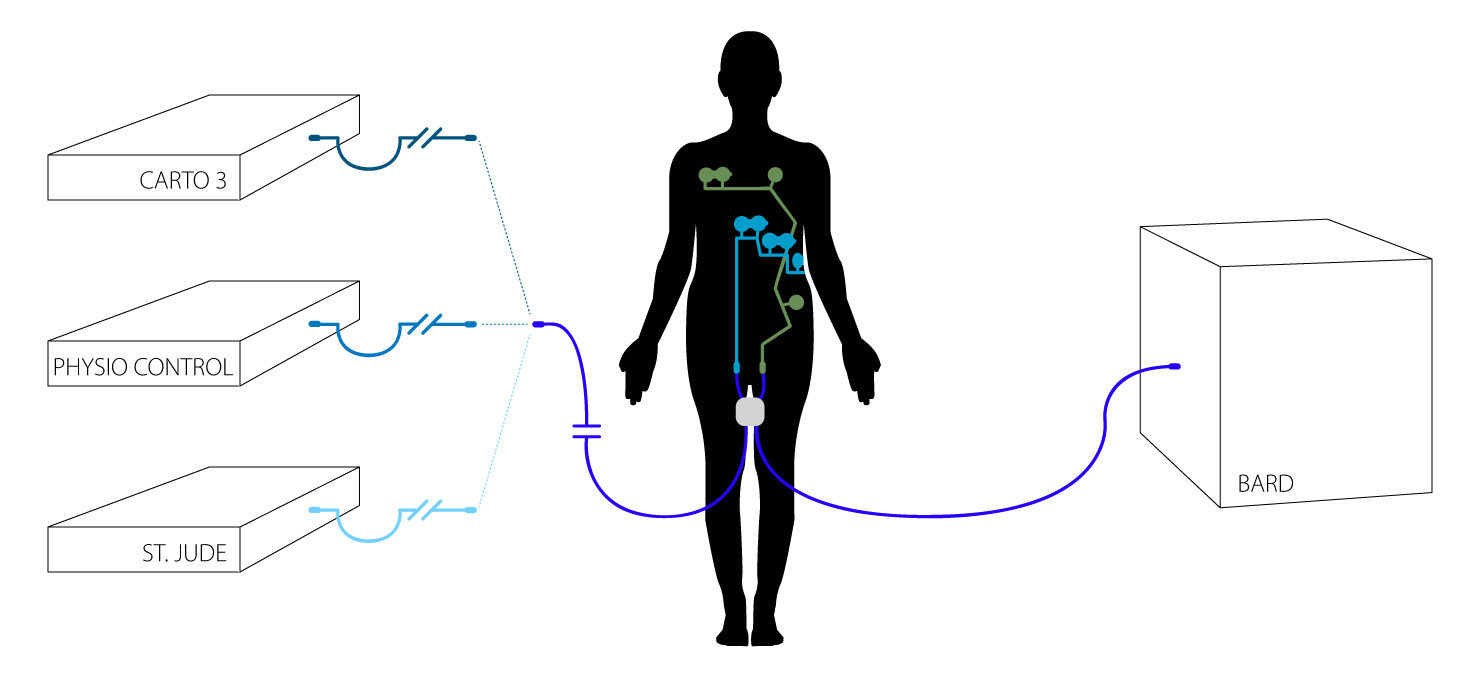
The CLARAVUE® system combines prewired, single-use, radiolucent ECG electrodes with our patented, unique Bioactive Integral Process trunk cables — consolidating leadwire sets into two combined connections with standardized compatibility to all monitors.
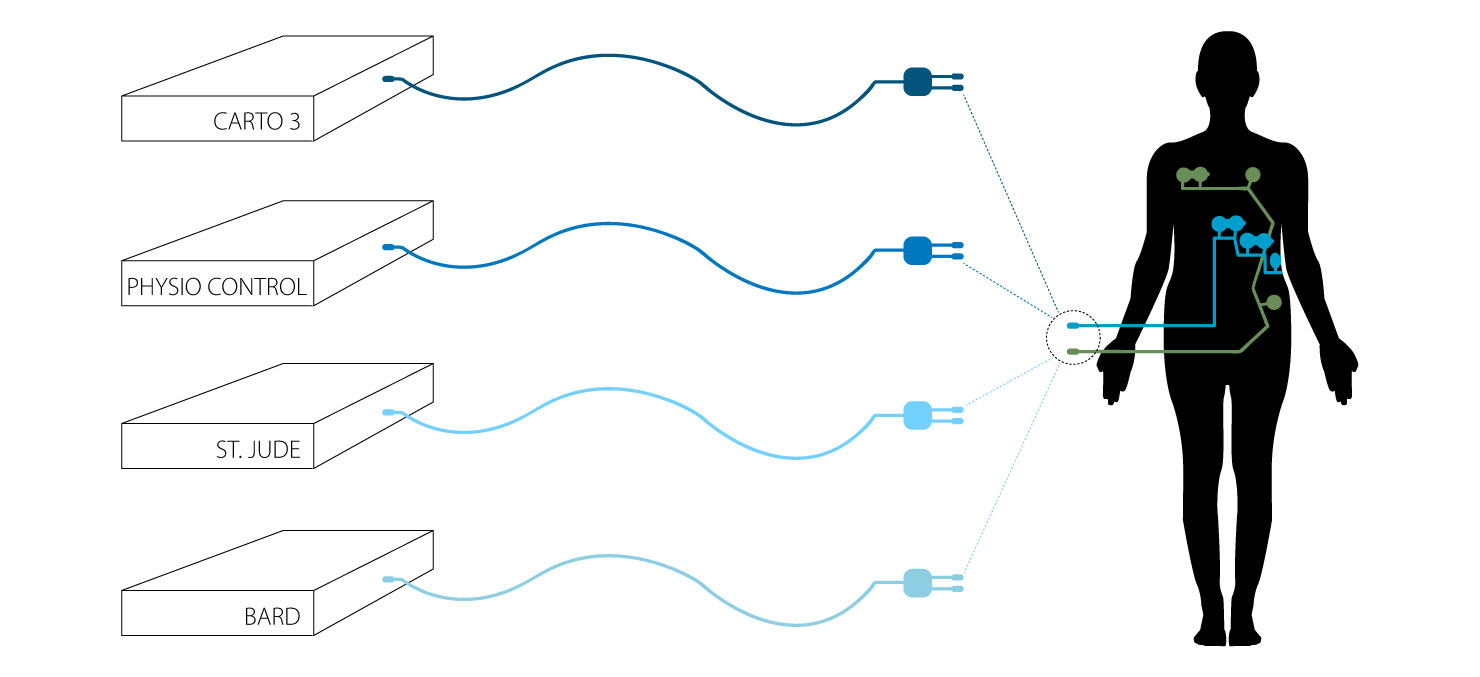
Multi-Parameter CLARAVUE® System
Compatible cables include a quick connect adapter, customizable to a variety of other equipment. Contact us for further information on simplifying your procedure setup using CLARAVUE®.
CLARAVUE Packaging
CLARAVUE comes packaged in a dispenser case designed for easy storage and efficient product retrieval. Each patient kit is a single-use pack, containing all prewired electrodes.


Instructions For Use
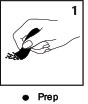
Prep
Standard protocol for skin prep applies.
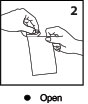
Open
Open the single CLARAVUE patient kit. Remove inner bag. Unwind sets from the holder card.
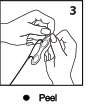 Prep
Prep
Gently remove electrode sets from release liner by peeling away from thumb tab.
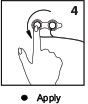
Apply
Adhere the RA and RL electrode positions to the patient. Continue to apply set A across the patient’s chest to the LA position and down to the LL position. Repeat with set B, applying the V positions from left to right. CLARAVUE is repositionable, allowing the freedom to rearrange on the patient as necessary. Note: Gently pull the wire from the ribbon cable to expand the overall size of the set(s).
Insert
While holding the male end of the adapter (clear clip) attached to set A, align with the blue, triangular indicator. Press firmly until lever snaps. Repeat with the blue clip attached to set B.
5 Day Wear
Ensure a good signal is achieved on the monitor. If a standard three lead trace is necessary, unplug set B from the adapter cable and remove the corresponding V positions from the patient. CLARAVUE’s various lead configurations enables total hospital standardization.
BioActive Cables
Coated with molybdenum trioxide (M0O3), CLARAVUE's unique blue trunk cables support prevention of healthcare-associated infections. The metalloacid coating produces a powerful antimicrobial effect designed to lower the contamination risks of standard non-coated cables.
In a recent study:
- Metalloacid coated surfaces “exhibited significant antimicrobial activity relative to that of the control surfaces within two to six hours after contact with the micro organisms.”
- This metalloacid material produces oxonium ions (H3O+), thus producing an acidic pH that is an effective antimicrobial.
- Post-exposure to eight multi-drug resistant bacteria strains, bacterial counts are significantly lower after 6-24 hours of contact.
- The total antimicrobial effectiveness is thought to be related to the H3O+ ion permeability of the cell membrane.
- In combination with employing regular cleaning procedures, “coated device surfaces may provide a permanent means of minimizing microbial contamination between two cleaning procedures.”
Nathalie Tetault, H. G.-H.-M. (2012). Biocidial activity of metalloacid-coated surfaces against multidrug-resistant microoganisms. Antimicrobial Resistance and Infection Control, 1-35.
Download Center
Nissha Medical Technologies offers additional literature and product
documents available to all users, for free in our download center.
More Information